
Minus K Educational Giveaway - June 2024
Low-Frequency Vibration Isolation Stabilizes Scanning Tunneling Microscopy Research of Nanoscopic Materials at Purdue University's Claridge Research Group
The Claridge Research Group, a nanotechnology research unit within Purdue University's Department of Chemistry, relies on Negative-Stiffness vibration isolation to provide stability for its nanometer-level research which employs scanning tunneling microscopy (STM). The STM, which incorporates microwave frequency bias modulation, is used to extract structural information from proteins and other complex molecules to optimize the performance of nanoscopic materials and
to better understand membrane protein structure.

Purdue Graduate student Emmanuel Nava loading and running their Pico 5500 AFM to obtain molecular-resolution images
Imaging molecules, materials and interfaces at nanometer lengths presents a challenge for nanoscience. Precisely controlling and characterizing interfacial structures at these length scales can be difficult. Organic and inorganic interfaces are a key determinant of nanomaterial optoelectronic properties and nanoscale device performance. They are also a determinant of structures of transmembrane proteins. Further, layered materials typically require noncovalent functionalization strategies that further complicate detailed chemical characterization of the interface
Claridge Research Group
It has been the challenge of Purdue University's Department of Chemistry, Claridge Research Group, to research and develop new integrated imaging strategies that probe the limits of interfacial ordering complexity and structural analysis at nanometer levels, addressing challenges ranging from optimizing the performance of nanoscopic materials to understanding membrane protein structure.
The group utilizes a wide variety of nanoscale analysis techniques, both within the laboratory and at the department's Analytical Instrumentation Center and Purdue's Birck Nanotechnology Center. These include scanning probe microscopies (such as atomic force microscopy and scanning tunneling microscopy), advanced surface analysis methods (such as polarization-modulated IR reflection absorption spectroscopy), and large-scale molecular modeling.
Employing these instrumentation techniques, the group's research has been addressing critical nanoscopic structures, including:
a) Patterning surface chemistry on the 5 -10 nm scale - to enable precisely controlled interactions with cell membranes, efficient exciton separation in organic photovoltaic devices, and molecular circuits mirroring the functional complexity observed in biology.
b) Development of custom nanoscale surface analysis instrumentation - to enable molecular-scale chemical imaging and characterization of dynamic processes at hydrophilic-hydrophobic interfaces relevant to nanoscopic materials and biology.
c) Unconventional applications of bioanalytical techniques - including characterization of nanoscale anisotropic wetting phenomena similar to those occurring in biological water and ion transport.d) Integrating molecular modeling and advanced interfacial characterization - developing detailed predictive understanding of noncovalently assembled interfaces with technologically important layered materials, such as graphene.
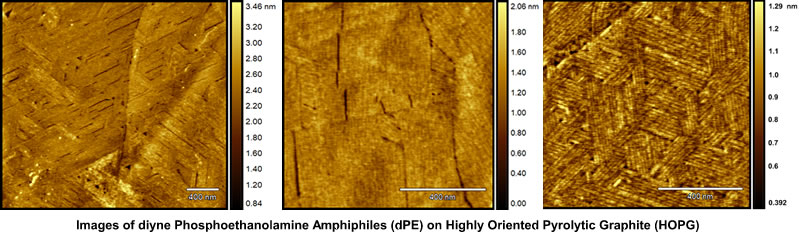
Imaging Nanoscopic Biological Monolayers
and Bilayers within Peptides
The Claridge Research Group has also been conducting research into the synthesis of novel polymerizable amphiphiles, such as peptides, and other molecules useful for noncovalent functionalization of layered materials.
Amphiphiles are chemical compounds possessing both hydrophilic and lipophilic properties, forming the basis for a number of areas of research in chemistry and biochemistry. Peptide amphiphiles are peptide-based molecules that self-assemble into high aspect-ratio nanofibers, capable of combining into various supramolecular structures.
"Our group uses both scanning tunneling microscopy (STM) and atomic force microscopy (AFM) for imaging small biological monolayers and bilayers, usually within peptides, the core components that make up proteins," said David G. McMillan with the Claridge Research Group. "These structures are not very well understood. We are trying to get a better understanding on the actual chemical makeup and behavior of these structures, at scales right around 7 - 14 nanometers. They are difficult to crystallize, so it is very hard to get crystallography data about them, which is how structures that small are normally characterized."
"A major research thrust in our group is the design of custom scanning probe instrumentation that provides structural resolution within single molecules at the sub-nanometer scale," added McMillan. "This is particularly relevant with our custom-built STM. By incorporating bias-modulation at the tunneling junction, and other custom modalities, we extract structural information from proteins and other complex molecules relevant to molecular electronics and human health."
Scanning Tunneling Microscopy
Scanning tunneling microscopy is based on the concept of quantum tunneling. When a conducting tip is brought very near to the surface to be examined, a bias (voltage difference) applied between the two can allow electrons to tunnel through the vacuum between them. The resulting tunneling current is a function of tip position, applied voltage, and the local density of states of the sample.
Information is acquired by monitoring the current as the tip's position scans across the surface, and is usually displayed in image form. STM can be a challenging technique, as it requires extremely clean and stable surfaces, sharp tips, sophisticated electronics, and excellent vibration control.
For an STM, good resolution is considered to be 0.1 nm lateral resolution and 0.01 nm depth resolution. With this resolution, individual atoms within materials are routinely imaged and manipulated. The STM can be used not only in ultra-high vacuum but also in air, water, and various other liquid or gas ambients, and at temperatures ranging from near zero kelvin to a few hundred degrees Celsius.
"Scanning tunneling microscopes provide atomic or molecular resolution imaging by precisely controlling the position of an atomically-sharp metal tip that is rastered across a sample, maintaining a separation of 1 nm between the tip and the sample," continued. McMillan. "Feedback is based on a 10 pA current of tunneling electrons, and even a 0.1 nm change in distance between the tip and the sample creates a large change in the tunneling current. Therefore, although the instrument is designed to maximize rigidity and minimize thermal drift, minimizing vibration, acoustic and electrical noise, and thermal drift are all imperative."
"Vibration and low-frequency acoustical noise are particularly critical, as the measured signal - the change in tip height as the atomically-sharp tip scans over a molecule on the surface - contains frequencies in the range from 10 - 400 Hz." explained McMillan.

Vibrations Influence STM Imaging
To achieve the nanometer-level imaging of the chemical makeup and behavior of these small biological monolayer and bilayer structures requires that the STM be positioned in an ultra-stable operating environment, one free of ambient low-frequency vibrations.
"Our lab is located on the fourth floor of a reinforced-concrete building with a column-beam design," said McMillan. "The presence of very large air handling equipment required for safety in our chemistry lab adds significant vibration in the structure. The building is always perpetually slightly moving. The structure's fundamentals are in the range 10 - 25 Hz, and the air handling equipment adds measurable noise at 14, 29 and 42 Hz. Long-term construction outside the building adds additional vibrations."
The STM is located in an extremely small room - 80 square feet, which offers the
lowest vibration levels available in the lab space due to its location near the building's structural supports. It is housed in an acoustical isolation/thermal-control chamber of modest size, optimizing use of the space. Payload weight - the STM, including its complete microwave setup - is approximately 25 pounds, with dimensions of
10" W x 10" D x 8" H.
Negative Stiffness Vibration Isolation
The Claridge Research Group made the decision to install Negative-Stiffness vibration isolation for its STM. Because of their very high vibration isolation efficiencies, particularly in the low frequencies, Negative-Stiffness vibration isolation systems enable vibration-sensitive instruments, such STM and AFM, to operate in severe low-vibration environments that would not be practical with top-performance air tables and other vibration-mitigation technologies.
Developed by Minus K Technology (www.minusk.com), Negative-Stiffness isolators employ a unique and completely mechanical concept in low-frequency vibration isolation. They do not require electricity or compressed air. There are no motors, pumps or chambers, and no maintenance because there is nothing to wear out. They operate purely in a passive mechanical mode.
"Vertical-motion isolation is provided by a stiff spring that supports a weight load, combined with a Negative-Stiffness mechanism," said Erik Runge, Vice President of Engineering at Minus K Technology. "The net vertical stiffness is made very low without affecting the static load-supporting capability of the spring. Beam columns connected in series with the vertical-motion isolator provide horizontal-motion isolation. A beam column behaves as a spring combined with a negative-stiffness mechanism. The result is a compact passive isolator capable of low vertical and horizontal natural frequencies and high internal structural frequencies."
Negative-Stiffness isolators achieve a high level of isolation in multiple directions. They have the flexibility of custom tailoring resonant frequencies vertically and horizontally to 0.5 Hz* (with some versions at 1.5 Hz horizontally). (*Note that for an isolation system with a 0.5 Hz natural frequency, isolation begins at 0.7 Hz and improves with increase in the vibration frequency. The natural frequency is more commonly used to describe the system performance.)
At 0.5 Hz there is almost no energy present. It would be very unusual to find a significant vibration at 0.5 Hz. Vibrations with frequencies above 0.7 Hz (where negative-stiffness isolators begin isolating) are rapidly attenuated with increases in frequency. When adjusted to 0.5 Hz, Negative-Stiffness isolators achieve approximately 93 percent isolation efficiency at 2 Hz; 99 percent at 5 Hz; and 99.7 percent at 10 Hz.
Vibration Isolation Efficiencies
"Negative-Stiffness isolators are exceptionally good at attenuating vibration in the range of 8 - 30 Hz, which has been problematic using other vibration isolation setups, continued McMillan. "In our experience, the low fundamental frequency of the Negative-Stiffness isolator (0.5 Hz) has improved attenuation at these proximal frequencies."
"The passive nature of the Negative-Stiffness vibration isolator frees us from having to provide additional penetrations and connections into our acoustical isolation chamber, eliminating possible sources of acoustical noise in the enclosure," added McMillan. "nanometersMinimizing the number of cables running into the enclosure also limits the extent to which cable vibrations can couple into the experiment, and possible additional sources of electronic noise."
The nature of nanometer-level research utilizing STM or AFM instrumentation necessitates a higher precision of vibration isolation than what can be achieved with high-performance air tables, as well as a higher level of environmental flexibility than what can be achieved with active isolation systems. Fortunately, for such precision research requirements, Negative-Stiffness delivers a compatible solution.
About the Claridge Research Group
A central theme of the Claridge Research Group, Department of Chemistry at Purdue University, is the development of new self-assembly and integrated imaging strategies that advance the limits of interfacial ordering complexity and structural analysis, addressing challenges ranging from optimizing the performance of nanoscopic materials to understanding membrane protein structure.
For more information contact David G. McMillan Claridge Research Group, Department of Chemistry, Purdue University 560 Oval Drive, West Lafayette, Indiana 47907-2084; Phone 765-494-5200; email dgmc@purdue.edu; www.purdue.edu.
|